

Reliable Probe Disinfection for Infection Prevention
The trophon 2 device enhances infection prevention protocols through automated high-level disinfection (HLD) for ultrasound probes in a closed system. Leveraging a 2ml dose of H202 in a nebulised form, each cycle achieves high level decontamination and overcomes the complex probe design shapes, as well as penetrates cracks, and cavities. Automation across the post cleaning disinfection part of the reprocessing workflow meets the healthcare needs for high standards of patient care and safety.
Automated processes reduce manual handling, ensuring reproducibility while reducing the chance for variability and errors. The trophon 2 device operates a simple RFID based user screen, with 30s hands on time to load the probe, freeing up clinical teams to focus on the patient or other tasks. A standby feature ensures readiness for immediate operation, even before clinics begin their workflows, enhancing overall efficiency further. The user-friendly interface supports ease of operation, while built-in prompts provide clear guidance. The 7-minute probe cycle integrates easily in daily schedules of 15+ minutes appointment times. Nanosonics supports staff education also and our team is on hand to run audited pre-installation training for users, as well as refresher training.
Integrated traceability tools provide clear documentation of every disinfection cycle, simplifying compliance with healthcare regulations. This digital record-keeping functionality minimises the administrative burden on clinical teams, allowing them to devote more time to patient-focused tasks. Additionally, reduced manual tracking contributes to a lower chance of record-keeping inaccuracies or oversights during audits.
The automated and hands off disinfection cycle of the trophon 2 device ensures consistent, reproducible outcomes each time. Once the probe is loaded, the device minimises reliance on manual intervention, reducing the chance of operator error. The closed operating system ensures that ultrasound probe is disinfected to high standards and in line with infection prevention guidelines as well as probe manufacturing IFUs and compatibility requirements.
Using hydrogen peroxide (H2O2) technology and ultrasonic vibration, the trophon 2 device creates a fine mist that ensures even the most difficult to reach surfaces are effectively disinfected. Once the cycle completed, the H2O2 breaks down into water and oxygen, which is safer for people, the equipment, and the environment. Using trophon technology also promotes positive outcomes in patient cross infection control.


trophon Technology Delivers on Probe Compatibility
Probe compatibility with any disinfection process is an important part of protecting equipment. Probe damage can have a range of causes, from dropping or knocking the probe to the use of incompatible reprocessing methods. Aggressive cleaning methods, such as vigorous wiping motions, can cause mechanical damage to probes. This can result in costly fixes or new purchases of technology may be required. To date, over 1,300 probes from 26 different manufacturers have been rigorously tested, approved, and endorsed for compatibility with trophon technology. That’s a whopping 123 working days per instrument assessed!
Find out more: Probe Care | Nanosonics
Simple and Efficient Operation
![]()
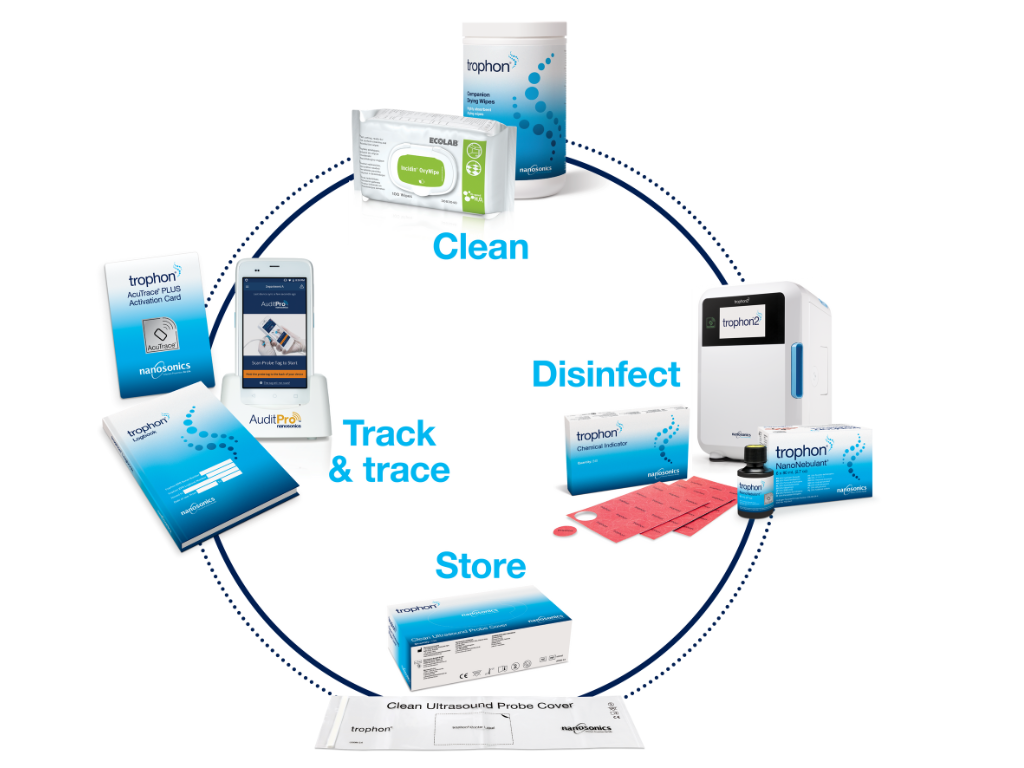
Total Reprocessing Solution
The Nanosonics Total Reprocessing Solution provides a comprehensive workflow for ultrasound probe reprocessing, covering every step from preparation to traceability.
Key features include:
trophon Companion Drying Wipes are highly absorbent, ensuring probes are completely moisture-free before and after disinfection.
Utilises proprietary NanoNebulant® disinfectant with AcuTrace® technology for effective high-level disinfection and record-keeping.
trophon Clean Ultrasound Probe Cover prevents recontamination post-disinfection.
Digital tools such as the Medical Instrument Tag and Operator Card capture and store reprocessing and operator details.
Automated compliance solutions like AuditPro link disinfection data to patient records, eliminating manual logbooks.
Why Nanosonics Leads the Way in Infection Prevention
Nanosonics is committed to supporting infection prevention through innovations that prioritise operational efficiency and environmental safety. With services like in-field validation, annual preventative maintenance, and system checks, healthcare providers can ensure continued reliability and compliance.
The trophon 2 device offers a wide range of tailored financing options and optional connectivity features to meet the needs of organisations of varying sizes and resources. This adaptability ensures that facilities can implement effective solutions for infection prevention while safeguarding patient care and the environment.
Nanosonics remains a trusted partner in delivering technologies designed to support the safety of healthcare professionals, patients, and medical equipment.
Speak to a trophon Specialist



