Every day, 106,000 patients are protected from the risk of cross contamination because the ultrasound probe has been high-level disinfected with the trophon® device.
Since 2009, the trophon devices have redefined the standard of care in ultrasound probe reprocessing providing fail-safe ultrasound probe high level disinfection (HLD) to protect patients from the risk of cross contamination.
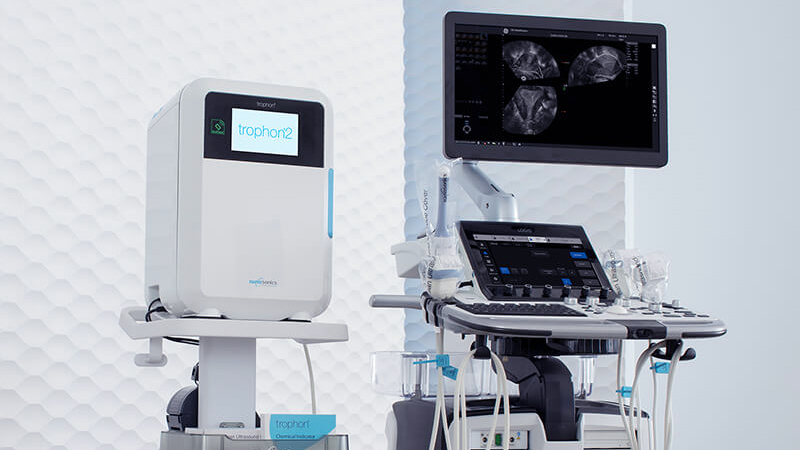
With the introduction of the trophon2 device, the world's best just got better. The trophon2 technology has an enhanced user experience for greater workflow efficiencies and traceability across the workflow for demonstrated compliance, to deliver consistent patient protection with every automated HLD cycle.

trophon technology is the only automated HLD technology for transvaginal, transrectal and surface probes to meet mandatory microbial efficacy test requirements for both CE mark and FDA registration.
In addition to mandatory testing, trophon technology is demonstrated to eliminate an extended range of clinically relevant pathogens including multi-drug resistant bacteria, blood borne viruses and sexually transmitted pathogens.
AcuTrace® RFID technology simplifies the creation of accurate digital records across the reprocessing workflow to support audit readiness.
With a trophon2 device and AcuTrace, users can capture the full reprocessing workflow electronically, removing the need for manual record keeping which is time consuming and error prone.
trophon2 devices are a fully enclosed, automated HLD system that saves you time with a simple, fast and integrated workflow solution. trophon2 devices makes point of care reprocessing possible, offering a solution that has been designed specifically for patient examination rooms.
trophon2 devices are simple to operate and with minimal training, new staff members can confidently deliver consistent HLD of your ultrasound probes. With new and improved features, the trophon2 device can be configured so it is always ready when you are for clinical workflows to be streamlined.
Nanosonics has implemented an industry leading compatibility program, which is conducted collaboratively with the ultrasound probe manufacturers and Nanosonics. Only probes approved by the probe manufacturers are listed as compatible with trophon devices.
Today, over 1,300 probes from all major and many specialty probe manufactures have been approved as compatible with trophon devices.
In addition to HLD, the trophon device portfolio offers a number of consumable and accessory product solutions to allow users to prepare, disinfect, track and trace, and store ultrasound probes therefore offering a complete reprocessing solution.
The trophon technology family includes trophonEPR devices and trophon2 devices which share the same core technology of 'sonically activated' hydrogen peroxide.
Find out how easy it is to integrate trophon2 devices into your practice
*The trophon® family includes trophon® EPR and trophon®2 which share the same core technology of sonically activated hydrogen peroxide.

